Start seeing your patients’
potential tumour drivers
more clearly

The molecular biomarkers and pathways involved in UC are key to understanding its biological heterogeneity and identifying specific subtypes, which may be used to predict treatment outcomes. In other words, knowing the molecular subtype of a given UC could help predict the clinical outcomes and treatment benefits for patients.

A focus on the distinct molecular subtypes in UC could help you see your individual LA/mUC patients more clearly
What’s more, understanding the molecular subtypes of bladder cancer can help reveal potential biomarkers, identify distinct patient subpopulations and inform treatment decision-making.
Some of the emerging, potentially predictive biomarkers for UC include:
> FGFR alterations
> PD-(L)1 expression
> Tumour mutational burden
> Tumour microenvironment
> Other molecular signatures

Discuss available biomarkers and related molecular testing options with your pathologist today to better understand your patients’ tumour growth drivers
The role of molecular subtypes in LA/mUC management
A detailed molecular understanding of UC has resulted in the identification of distinct subtypes that could help guide treatment approaches. For example, molecular subtypes of MIBC have been associated with different responses to treatments such as chemotherapy or immunotherapy.
More specifically, recent developments in our understanding of the pathology of bladder cancer have led to the identification of six molecular classes. In descending order of prevalence, the six subtypes of MIBC are:

Basal/squamous (35%)

Stroma-rich (15%)

Luminal papillary (24%)

Luminal non-specified (8%)

Luminal unstable (15%)

Neuroendocrine-like (3%)
As shown in the table below, each molecular subtype of MIBC has distinct:
> Differentiation patterns (e.g. urothelial/luminal, basal or neuroendocrine)
> Oncogenic mechanisms (e.g. FGFR3, PPARG, RB1, etc.)
> Tumour microenvironments (e.g. stromal or immune infiltration)
> Histological characteristics (e.g. papillary, squamous or neuroendocrine)
> Clinical associations (e.g. tumour stage, sex or age)
Adapted from Kamoun A et al. 2020.

In certain types of cancer, an improved understanding of molecular pathology has enabled the arrival of precision medicine, which can help improve treatment outcomes for patient subpopulations with the appropriate biomarkers
The current approach to LA/mUC management
A bladder cancer patient’s chance of survival is highly stage-dependent, and progression from MIBC to metastasis is common
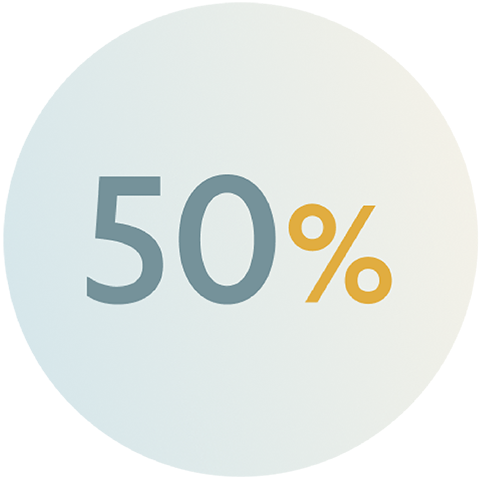
Despite guideline-recommended radical cystectomy, up to 50% of patients with MIBC may experience distant recurrence.
Additionally, systemic recurrence is more common in locally advanced disease (ranging from 32-62%) as well as in patients with lymph node involvement (ranging from 52-70%).
The treatment approach in LA/mUC remains controversial, with evidence about some areas of bladder cancer management being limited and/or conflicting

The treatment landscape for bladder cancer is beginning to experience a paradigm shift towards the use of targeted therapy, owing to the following:
> a better understanding of the molecular and genetic heterogeneity of tumour types
> the investigation of molecular signatures predicting disease progression and therapeutic response
> the discovery and implementation of tailored therapies

However, molecular biomarkers are not commonly used in routine clinical practice for LA/mUC patient care.

Knowing the molecular profile of your patients’ tumours could inform treatment decision-making, helping to improve patient outcomes
Genetic testing in advanced or metastatic tumours
Understanding the genomic alterations that can drive tumour growth has improved patient outcomes across cancer types. As a consequence, international guidelines recommend the routine use of molecular testing in advanced or metastatic tumours, owing to its potential benefits for oncology patient care.

- The ESMO guidelines recommend the routine use of NGS in non-squamous non-small cell lung, prostate, ovarian and cholangiocarcinoma advanced cancers.
- The NCCN® Bladder Cancer Panel recommends that molecular/genomic testing be performed ideally upon diagnosis of LA/mUC to inform treatment decision-making and navigate through later lines of treatment.

Optimised molecular testing protocols could enable the timely identification of relevant genetic alterations that could be driving your patients’ tumour growth
The value of MDTs in genetic testing
The management of UC is increasingly becoming multidisciplinary, with close cooperation and critical input needed from different specialities to inform effective disease management plans for patients.
Work with your pathologist now to set up a testing infrastructure and/or ensure your testing capabilities cater to the advancement of biomarker-led precision medicine for your LA/mUC patients

- A pathologist is a vital partner when diagnosing UC and identifying the type of tumour present, which is critical to tailoring treatment plans for LA/mUC patients and achieving optimal clinical outcomes.
- As the turnaround time for different NGS platforms may range from ~2 hours to 12 days, make sure to perform molecular testing upon diagnosis of advanced disease in order to inform treatment decision-making and develop a personalised treatment plan for your LA/mUC patients.
When establishing molecular testing protocols for your LA/mUC patients, it is important to liaise with the treating urologist to ensure sufficient sample quality is available
- Specimen quality, including tumour cellularity, preservation and fixation status, is critical for optimal molecular testing practices.
- FFPE specimens are normally used for most molecular testing techniques due to the advantage of preserved morphological details and the ability to store samples long term and at low cost.
- It is not always possible to acquire large quantities of DNA for genetic testing; however, NGS methods require nanogram quantities of DNA compared with traditional Sanger sequencing, which requires microgram quantities.


When requesting molecular testing, make sure to consider the total turnaround time and liaise with your MDT to ensure you have the results to inform treatment decision-making and navigate through later lines of treatment
APOBEC: apolipoprotein B mRNA editing catalytic polypeptide-like; CD8 T cells: cytotoxic T lymphocytes; CDKN2A: cyclin-dependent kinase inhibitor 2A; DNA: deoxyribonucleic acid; E2F3: gene encoding E2F transcription factor 3; EGFR: epidermal growth factor receptor; ELF3: gene encoding E74-like ETS transcription factor 3; ERBB2: gene encoding receptor tyrosine-protein kinase erbB-2; ERCC2: gene encoding XPD protein; ESMO: European Society of Medical Oncology; FFPE: formalin fixed paraffin embedded; FGFR: fibroblast growth factor receptor; KDM6A: gene encoding lysine-specific demethylase 6A; LA: locally advanced; MDT: multidisciplinary team; MIBC: muscle-invasive bladder cancer; mUC: metastatic UC; NCCN®: National Comprehensive Cancer Network®; NGS: next-generation sequencing; NK cells: natural killer cells; PD-(L)1: programmed death-ligand 1; PPARG: peroxisome proliferator-activated receptor gamma; RB1: gene encoding tumour suppressor retinoblastoma protein 1; T2: tumour growth into muscle; T3: tumour growth into fat layer; T4: tumour growth outside of the bladder; TMB: tumour mutational burden; TP53: gene encodes p53; UC: urothelial carcinoma.